Life survives, and often thrives, across a wide range of environments from temperate to inhospitable. Many environments cycle between different states. Day leads to night. Winter follows summer. Monsoons transition to droughts. Throughout evolutionary history, adaptations have arisen to enable organisms to live across extreme environmental changes.
How do single cells defend themselves from harsh environments? What sort of molecular adaptations allow cellular processes to function under such varied conditions? Can we use what we learn to engineer living systems to be more resilient? Can we identify sustainable materials that protect biologics from environmental stress?
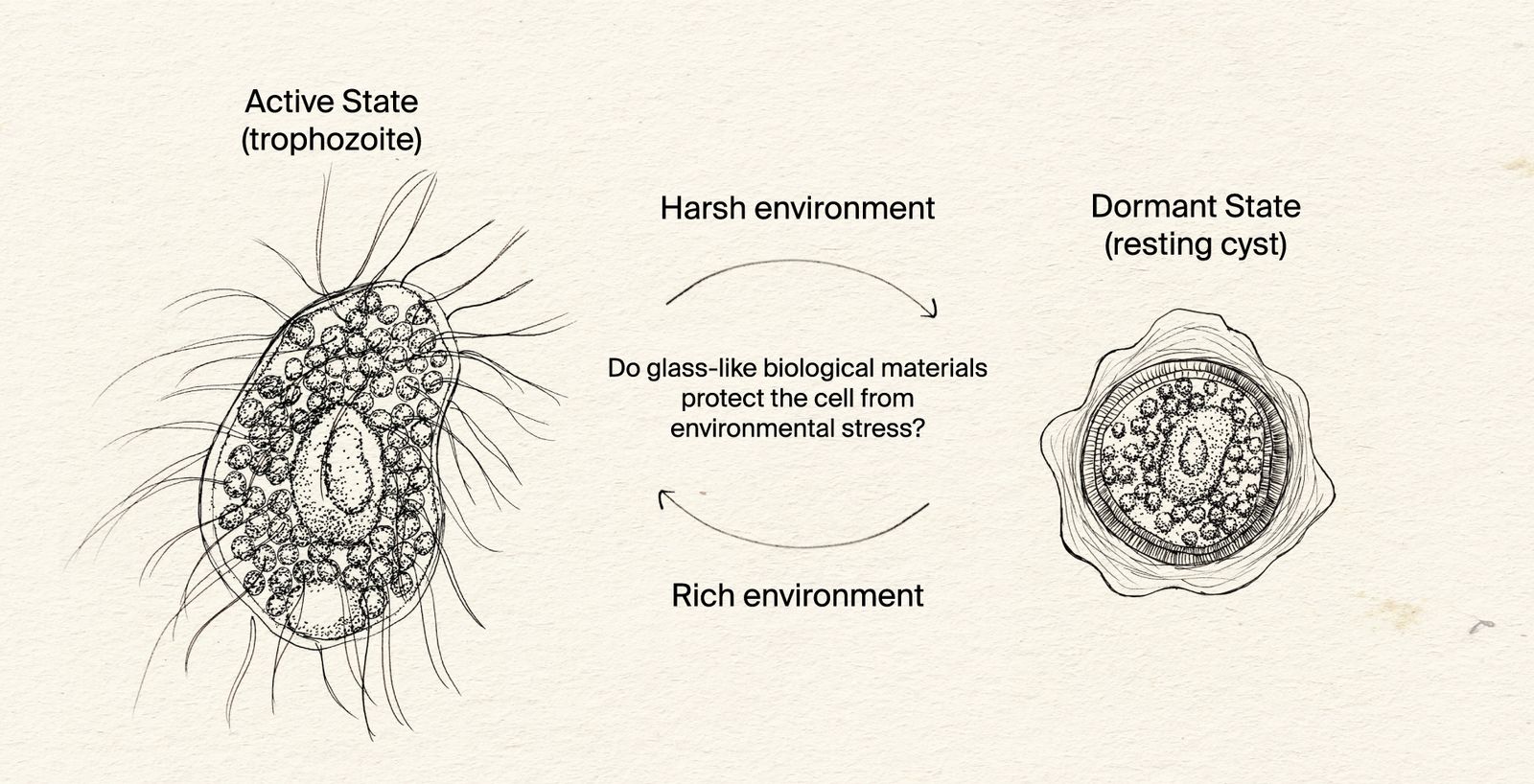
How does encystment protect protists from harsh environments?
Our initial focus is on Colpoda steinii, pictured here, a single-celled eukaryote that can be found in ephemeral bodies of water, such as dew that forms on plants, and creeks that dry up. In the active state, Colpoda steinii swims and feeds. Upon starvation, Colpoda forms a resting cyst that is environmentally resistant.
We seek to understand how organisms are adapted to live across extreme environmental changes. We focus our attention on simple and understudied systems, beginning with microscopic eukaryotes that protect themselves by forming a ‘cyst’ enclosed within a unique cell wall. To survive harsh environments, many eukaryotes become dormant in a so-called ‘resting cyst.’ The cyst’s specialized cell wall shields the organism from environmental stressors until conditions improve, at which point the organism emerges from the cyst.
Three questions about this process guide our work:
- What is the composition of the resting cyst wall and how is it built?
Resting cyst formation involves drastic morphologic, metabolic, and compositional changes. Protists shed the surface appendages that they use for motility. The organism spends energy to build the cell wall, and the cells, presumably, hoard energy to prepare for a future reanimation. Secretion is revved up to build multiple, distinct, layers of the cell wall. We are working to identify the molecular steps of these events. We predict that molecules in the cyst wall with glass-like properties protect the cell from environmental stress.
- Which stimuli reanimate cells from the resting cyst?
The emergence of the organism from the cyst poses conflicting challenges that need to be balanced. The organism must be dormant and protected enough to withstand prolonged exposure to adverse conditions. At the same time, the organism must also be competent enough to sense when the local environment becomes hospitable. The cyst wall shields the organism from harsh environments but remains permeable to reanimation signals. We are in pursuit of the specific molecules that are able to pass the cyst’s defenses and reanimate the organism.
- What is the evolutionary history of the resistance of cysts to desiccation?
Encystment is observed in many species across the eukaryotic tree of life. There is striking cyst diversity between species and within a species. We are leveraging an evolutionary perspective to identify similarities and differences in encystment and environmental adaptation between organisms. Integrating molecular information with knowledge of cyst-related phenotypes of Colpoda steinii and related organisms can help us make better hypotheses about the mechanisms of encystment and reanimation.
There are many types of environmental changes to which organisms are adapted, such as light to dark, hot to cold, and nutrient-rich to nutrient-poor. Wet to dry is a particularly fascinating environmental change, because desiccation is fundamentally antagonistic to life. Water is the most abundant molecule in cells, making up 70% of the cell’s mass
At the global scale, fresh water is perhaps the most important natural resource. Climate change and non-sustainable water usage are putting enormous strain on freshwater supplies
Despite the harshness of desiccation, many organisms are able to survive in extremely dry environments. Colpoda species are protists that live in ephemeral bodies of water, such as dry creeks, or even the dew that transiently forms on plants
We want to be able to observe Colpoda steinii encystment and excystment under different conditions, but immediately struggled to image the cells for the hours-long periods needed because they are motile and readily swim out of focus. Also, in pilot encystment assays there were often fewer cysts visible than predicted, suggesting that cells were encysting in locations invisible to the microscope, such as on the walls of the chamber.
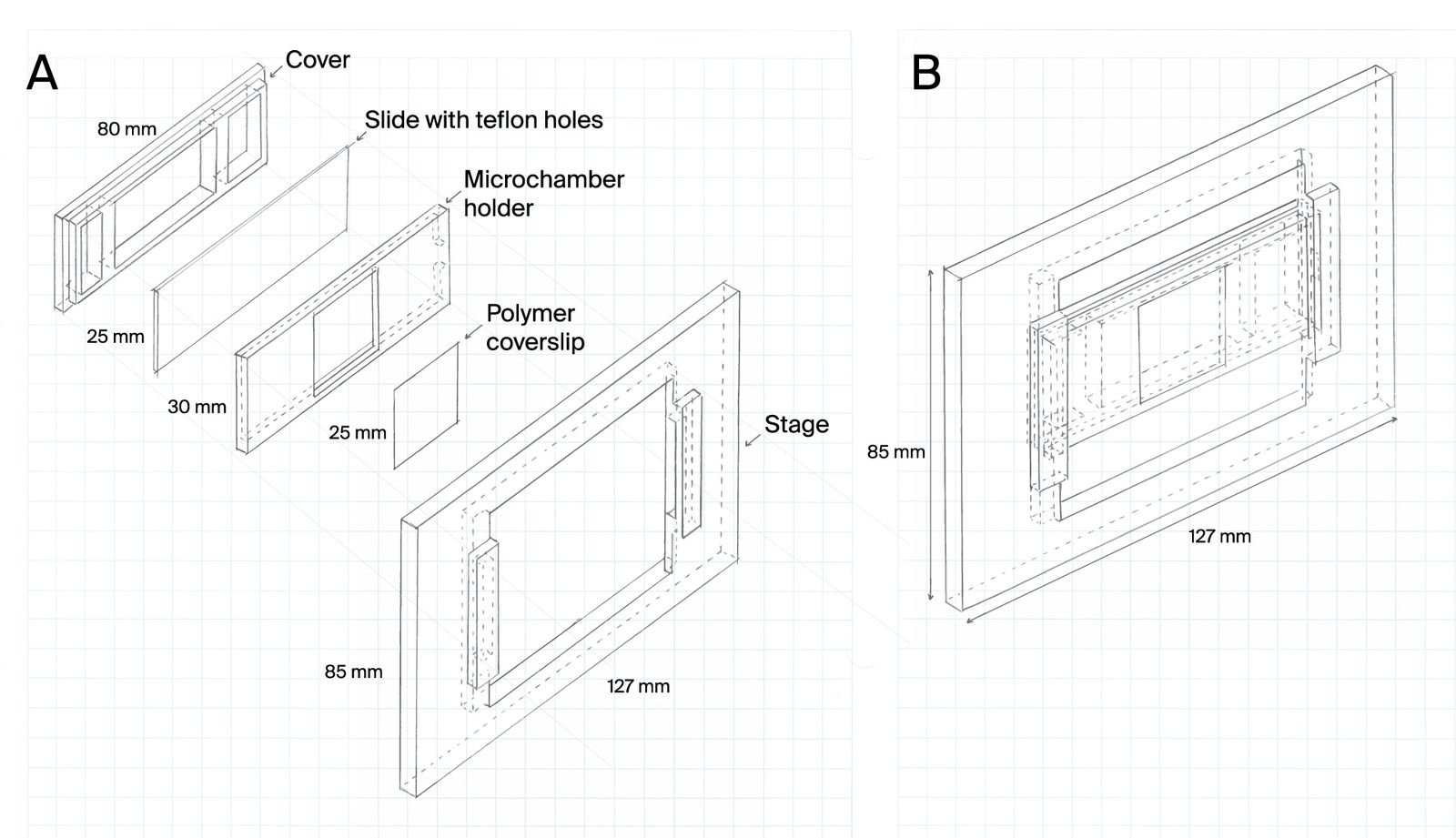
To address this, we developed microchambers that confine cells within the field of view. The microchambers enhance the probability that a cell will be in the field of view at any given time and ensure that cells encyst in locations that are visible under the microscope.
Learn more about this resource and try it yourself:
Microchamber slide design for cell confinement during imaging
Cells can be highly motile, moving in and out of a microscope’s field of view. Understanding complex life cycles is difficult without continuous observation. To overcome this challenge, we’ve developed a 3D-printed microchamber device to confine cells for long-term visualization.
We ultimately realized that we could take this work in a variety of directions, but none had immediately actionable translational implications. Our company strategy has shifted to both building platform tools that let us find useful innovation across the tree of life and rapidly testing out ideas for translational potential. We felt that we wouldn’t be able to iterate quickly with this project, so we decided to ice it.